Can Modafinil Cause Stevens-Johnson Syndrome?
Modafinil and its derivative, Armodafinil, are commonly prescribed to treat excessive sleepiness caused by conditions like narcolepsy and obstructive sleep apnea. While these medications are effective for improving wakefulness, concerns have emerged about a possible link to Stevens-Johnson Syndrome (SJS), a rare but serious skin reaction. Understanding this connection, backed by clinical reports and studies, is essential for safe use.
Understanding Modafinil and Armodafinil
Modafinil and Armodafinil are medications designed to promote wakefulness. They are commonly prescribed for:
- Narcolepsy: A chronic sleep disorder characterized by overwhelming daytime drowsiness and sudden attacks of sleep.
- Obstructive Sleep Apnea: A condition where the upper airway becomes blocked repeatedly during sleep, reducing or completely stopping airflow.
- Shift Work Sleep Disorder: A condition that affects people who work nontraditional hours, causing difficulty in maintaining a regular sleep schedule.
These medications work by altering the natural chemicals (neurotransmitters) in the brain, particularly those involved in the sleep-wake cycle. Despite their effectiveness, Modafinil and Armodafinil are not without risks, and their safety profiles need careful consideration.
What is Stevens-Johnson Syndrome (SJS)?
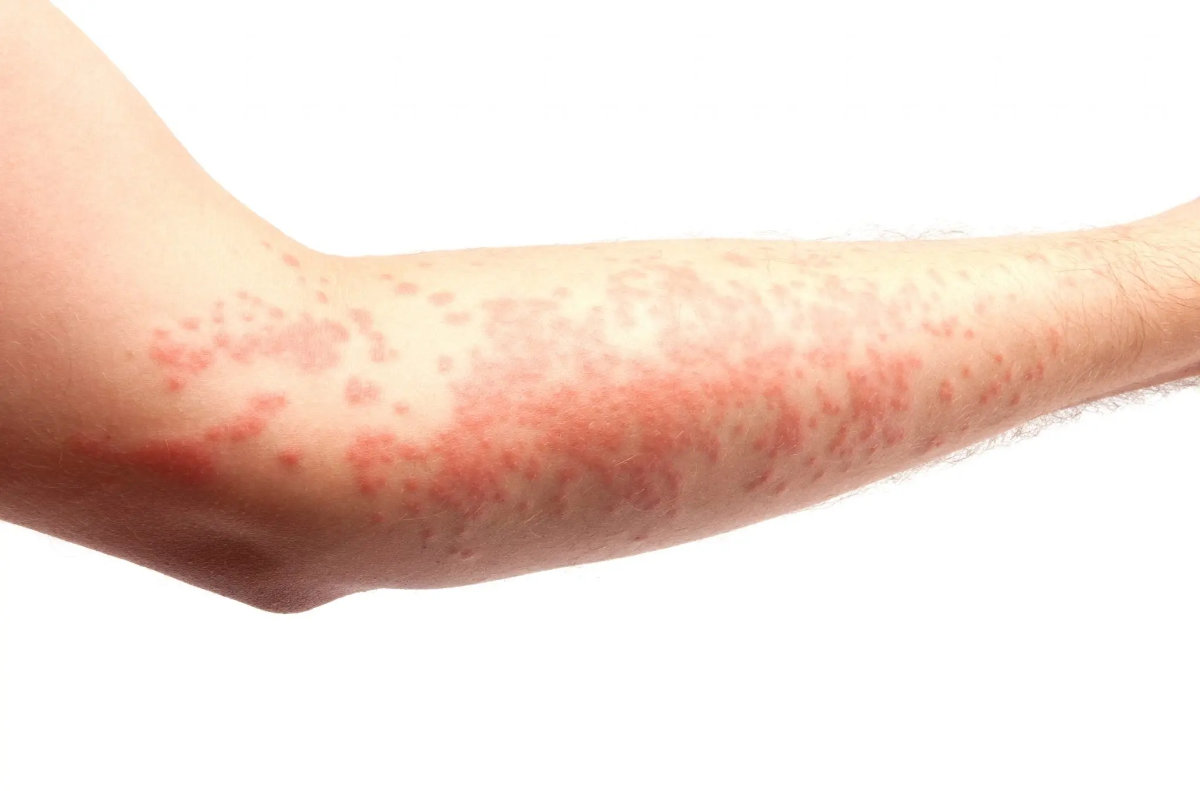
Stevens-Johnson Syndrome (SJS) is a severe and potentially life-threatening skin reaction. It is usually triggered by medications or infections and is characterized by:
- Flu-like symptoms such as fever and sore throat.
- Painful red or purplish rash that spreads and blisters.
- Shedding of the skin, revealing raw, painful areas.
SJS often requires hospitalization and can lead to severe complications, including infections, vision loss, and damage to internal organs. Prompt recognition and treatment are crucial for improving outcomes.
Clinical Cases and Reports
Case Reports from Literature
Several case reports have highlighted the link between Modafinil/Armodafinil and SJS:
- 21-Year-Old Woman: A 21-year-old woman developed SJS 12 days after starting Armodafinil. Her symptoms included a mild fever, neck swelling, mucocutaneous ulcerations, and a generalized rash. A skin biopsy confirmed the diagnosis of SJS. Despite discontinuing the medication and receiving supportive care, she experienced significant discomfort and skin discolouration.
- 40-Year-Old Woman: Another case involved a 40-year-old woman who developed SJS after three weeks of taking Modafinil. She presented with a sore throat, mouth pain, dysuria, and a widespread rash. Biopsy results showed full-thickness epidermal necrosis, leading to a confirmed diagnosis of SJS.
Findings from the Singapore Health Sciences Authority (HSA)
The HSA has received several adverse event reports related to Modafinil and Armodafinil, including cases of SJS. As of October 2023, nine adverse event reports were documented, with seven being dermatological reactions and three confirmed as SJS. These cases involved individuals who had obtained the medications from unauthorized sources, highlighting the risks associated with self-medication and unregulated drug use.
Incidence and Severity in Clinical Trials
Clinical trials have shown that the incidence of serious skin reactions, including SJS, is rare but notable. Reports indicate that the incidence of rash leading to drug discontinuation in pediatric patients under 17 years old was approximately 0.8%. Post-marketing surveillance has identified additional cases of SJS and other severe skin reactions, emphasizing the need for ongoing monitoring and vigilance.
Mechanisms and Pathophysiology
The exact mechanisms by which Modafinil and Armodafinil might trigger SJS are not fully understood. However, it is believed to be an immune-mediated process related to drug metabolites. Some key points include:
- Immune Response: The body’s immune system may react abnormally to the drug, leading to widespread skin and mucous membrane damage.
- Genetic Predispositions: Certain genetic factors, such as specific human leukocyte antigen (HLA) types, may increase susceptibility to SJS.
- Other Risk Factors: Conditions like HIV, lupus, and rapid drug introduction or high doses may also elevate the risk of developing SJS.
Understanding these mechanisms is crucial for identifying at-risk individuals and preventing severe adverse reactions.
Symptoms and Diagnosis
Recognizing the symptoms of SJS early can be life-saving. Common symptoms include:
- Prodromal Symptoms: Flu-like symptoms such as fever, sore throat, and general malaise.
- Skin and Mucosal Lesions: Painful red or purplish rash that spreads, blisters, and causes the skin to shed. Mucosal involvement often includes painful sores in the mouth, eyes, and genital areas.
Diagnosis is typically confirmed through a skin biopsy, which reveals full-thickness epidermal necrosis and subepidermal blistering. Immediate discontinuation of the suspected drug and supportive care are critical steps in managing SJS.
Regulatory and Safety Alerts
Health authorities worldwide have issued several warnings and safety alerts regarding the use of Modafinil and Armodafinil. These alerts emphasize the potential for severe dermatologic reactions, including SJS and Toxic Epidermal Necrolysis (TEN).
Drug Label Warnings
Both Modafinil and Armodafinil come with warnings about the risk of serious skin reactions. The labels highlight the importance of monitoring for early signs of these reactions and recommend immediate discontinuation of the drug if symptoms appear. Despite these warnings, many healthcare professionals remain sceptical due to the rarity of reported cases.
HSA Alerts
The Singapore Health Sciences Authority (HSA) has been proactive in issuing press releases to inform healthcare professionals and the public about the risks associated with Modafinil and Armodafinil. The HSA has reported cases of SJS and other severe skin reactions, particularly stressing the dangers of obtaining these medications from unauthorized sources.
Global Post-Marketing Surveillance
Post-marketing surveillance data has identified additional cases of severe skin reactions worldwide. These findings underscore the necessity for healthcare providers to be vigilant and for patients to be fully informed about the potential risks.
Management and Treatment of SJS
When SJS is suspected, immediate medical attention is required. The primary steps in managing SJS include:
Discontinuation of the Suspected Drug
The first and most critical step is to stop taking the medication believed to be causing the reaction. Early discontinuation is essential to prevent further progression of symptoms.
Supportive Care
Patients with SJS often require hospitalization, sometimes in an intensive care unit or burn unit, due to the severity of their symptoms. Supportive care involves:
- Fluid Replacement: To counteract dehydration from skin loss.
- Pain Management: To alleviate the significant discomfort associated with skin lesions and mucosal involvement.
- Infection Prevention: Use of antibiotics to prevent secondary infections from skin lesions.
Long-Term Management
After the acute phase, patients may need long-term care to manage complications such as skin scarring, vision problems, and psychological impact. Follow-up with dermatologists, ophthalmologists, and mental health professionals is often necessary.
Preventive Measures and Recommendations
To reduce the risk of SJS associated with Modafinil and Armodafinil, several preventive measures can be taken:
Guidelines for Healthcare Providers
Healthcare providers should conduct thorough patient assessments before prescribing these medications. This includes:
- Evaluating Patient History: Checking for any history of drug allergies or previous adverse reactions.
- Educating Patients: Informing patients about the signs and symptoms of SJS and advising them to seek immediate medical attention if they occur.
Consideration of Alternative Treatments
For patients at higher risk of adverse reactions, healthcare providers may consider alternative treatments for excessive somnolence. This could include other medications with a lower risk profile or non-pharmacological approaches.
Importance of Reporting Adverse Events
Healthcare professionals should report any adverse events related to Modafinil and Armodafinil to relevant health authorities. Accurate reporting helps in monitoring drug safety and can lead to better patient outcomes through improved safety measures and guidelines.
Conclusion
The potential link between Modafinil, Armodafinil, and Stevens-Johnson Syndrome is an important consideration for both healthcare providers and patients. While the incidence of SJS is rare, its severity necessitates a high level of vigilance and prompt action at the first sign of symptoms. Ensuring patient safety involves a thorough assessment, patient education, and adherence to regulatory guidelines. Further research and ongoing post-marketing surveillance are essential to fully understand and mitigate the risks associated with these medications.